Community Update Survey
Every individual with spinal muscular atrophy (SMA) and their families bring a unique perspective that collectively help us drive further progress and adapt to the changing landscape of SMA.
About the Survey
Since 2017, the Cure SMA Community Update Survey has collected data and information on the SMA community’s experiences and daily challenges. Our top priority is to represent the voice of the whole community so we can drive research and care to meet the needs of everyone impacted by SMA. Completing this survey is one tangible, and extremely valuable, way for you to make your voice heard.
Every piece of data collected allows us to track changes in the attitudes, feelings, unmet needs, and actions of the SMA community over time. We are grateful for the 2000+ community members who have completed at least one annual Community Update Survey!
Please see below for how your data has impacted the Cure SMA Community!
Clinical Trials and Care
Results from the Community Update Survey are used to inform and develop Cure SMA initiatives supporting clinical trials and care. Results from the Community Update Survey were included in the Critical Path Innovation Meeting. This meeting—held virtually in the summer of 2020—enhanced the U.S. Food and Drug Administration’s understanding of the most significant unaddressed needs for treating children and adults with SMA. Data from the Community Update Survey was also used in the Patient-Led Listening Sessions with the FDA. You can find out more about those sessions here.
Advocacy
Data from the survey has informed Cure SMA’s advocacy agenda and has been featured in legislative statements, and educational outreach to federal and state leaders to showcase the priorities and needs of the SMA community related to newborn screening, employment, transportation, community living, healthcare, and unmet needs.
Education and Awareness
Data from the Community Update Survey is presented in the annual Cure SMA State of SMA report. This annual data report describes the quickly changing landscape of SMA and highlights the current unmet needs of the community. Every year the report is shared with our families, chapter leaders, healthcare providers, and industry partners to promote awareness for the development of new programs and therapies. Because of your participation in the annual Community Update Survey we are able to capture the following changes:
- The increase in the number of individuals with SMA living into adulthood
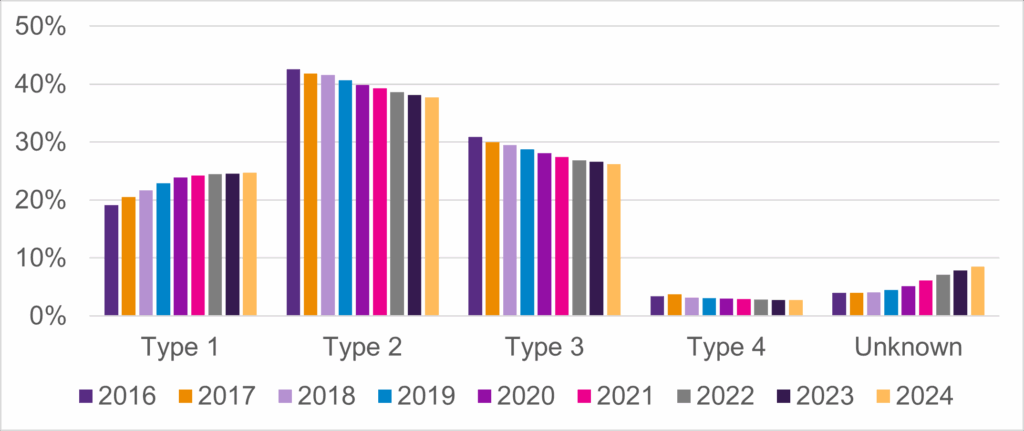
- The changing prevalence of SMA type (Figure 1)
- The community’s use of SMA treatments
- The prevalence of mental health issues among the SMA community
- Pregnancy rates among women living with SMA
- Shifting comorbidities
- The decreasing mortality rate
- And so much more
Figure 1: Prevalence of SMA Type Over Time
Funding for this research was provided by the Cure SMA Industry Collaboration.
About the Cure SMA Industry Collaboration
The Cure SMA Industry Collaboration (SMA-IC) was established in 2016 to leverage the experience, expertise, and resources of pharmaceutical and biotechnology companies, as well as other nonprofit organizations involved in the development of spinal muscular atrophy (SMA) therapeutics to more effectively address a range of scientific, clinical, and regulatory challenges.
Frequently Asked Questions
The 2025 survey will be open from April 21- May 12, 2025.
Cure SMA will email a survey link to all eligible individuals in the Cure SMA membership database. If you are eligible and have not received an email, please check your spam inbox or complete this form to request a survey link be emailed to you.
All personal information and individual responses will be kept confidential.
Please feel reach out to the Cure SMA survey team at [email protected].
Publications
Belter L, Jarecki J, Reyna SP, et al. Journal Neuromuscular Dis. 2021;8(1):109-123. doi:10.3233/JND-200563
Belter L, Cruz R, Jarecki J. Orphanet J Rare Dis. 2020 Aug 24;15(1):217. doi: 10.1186/s13023-020-01498-2
Peterson IS, Belter LT, Curry MA, Jarecki J. Telemed J E Health. 2024 Feb;30(2):536-544. doi: 10.1089/tmj.2023.0293



