Drug Discovery
Drug discovery takes what we’ve learned about the causes and biology of spinal muscular atrophy (SMA) and utilizes it for basic research into new drug candidates for testing in clinical trials.
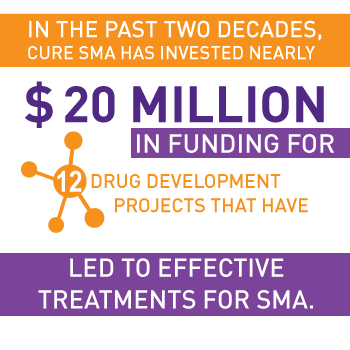
Our Achievements in Drug Discovery
Our diverse and broad-based approach has led to important breakthroughs, including the FDA approval of three treatments for SMA:
December 2016
The FDA approved Spinraza®, a treatment developed by Biogen and Ionis, making it the first-ever approved therapy for all types of SMA. Cure SMA provided the very first research funding for this program beginning in 2003.
May 2019
The FDA announced that it had approved Zolgensma®, a gene therapy developed by Novartis Gene Therapies and approved to treat children with all types of SMA under two years of age. Cure SMA provided funding for this program beginning in 2010.
August 2020
The FDA announced the approval of Evrysdi®, a treatment developed by Genentech, a member of the Roche Group, approved for the treatment of SMA in all ages and all types.
These approvals are just the leading edge of a robust drug pipeline, with a breadth and depth that reflects our goal of treatments for all ages and types. In addition to approved therapies, approximately 20 other programs are in development, including several in clinical trials.
We have identified a variety of therapeutic approaches that show promise in treating SMA.
Important biotech and pharmaceutical partners are committed to SMA research. Today, more than a dozen companies are engaged in SMA research.
Our Approach to Drug Discovery
Drug discovery can be a long and complex process, and it is difficult to predict which drugs will be successful. An estimated 90 percent of experimental drugs starting in human clinical trials never gain U.S. Food and Drug Administration (FDA) approval.
Our approach to funding spinal muscular atrophy (SMA) research minimizes common challenges of drug discovery, building a diverse “pipeline” of drug candidates:
We invest in many projects at once, which means if a drug candidate fails, several others can take its place
Our projects represent many therapeutic approaches, attacking all sides of spinal muscular atrophy (SMA)
We provide seed funding for new projects. Traditionally, it has been difficult to get pharmaceutical companies to invest in rare disease research. By providing early-stage funding, we lower the risk and attract larger investments from industry and government as drug candidates move through the process
We are unbiased as we evaluate all possible treatment opportunities, and we prioritize, select, and manage our drug discovery projects through a Translational Advisory Council, comprised of industry experts



