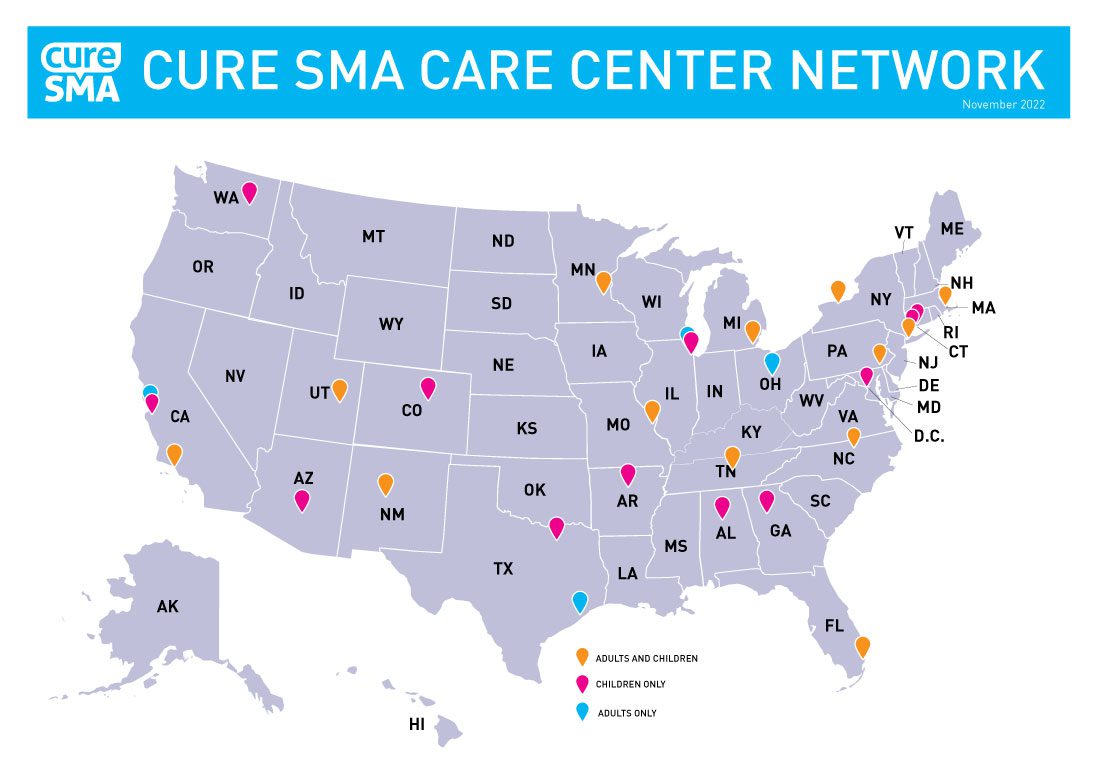
Cure SMA Care Center Network
The mission of the Cure SMA Care Center Network is to provide the best healthcare including offering new therapies and to gather and disseminate new knowledge to advance standard of care for pediatric and adult persons with spinal muscular atrophy (SMA).
Jump Links
Cure SMA Care Center Network Sites
- Boston Children’s Hospital, Boston, MA
- Columbia University, New York, NY
- Connecticut Children’s Medical Center, Hartford, CT
- Duke University Medical Center, Durham, NC
- Gillette Children’s Specialty Healthcare, St. Paul, MN
- The Children’s Hospital of Philadelphia, Philadelphia, PA
- The University of Michigan, Ann Arbor, MI
- University of California, Los Angeles (UCLA), Los Angeles, CA
- University of Miami, Miami, FL
- University of New Mexico, Albuquerque, NM
- University of Rochester Medical Center, Rochester, NY
- University of Utah, Program for Inherited Neuro Disorders, Salt Lake City, UT
- Washington University/St. Louis Children's Hospital, St. Louis, MO
- Baylor College of Medicine, Houston, TX
- Northwestern University, Evanston, IL
- Stanford Health, Palo Alto, CA
- The Ohio State University, Wexner Medical Center, Columbus, OH
- Advocate Children’s Hospital, Park Ridge, IL
- Arkansas Children’s Hospital, Little Rock, AR
- Children’s Healthcare of Atlanta, Atlanta, GA
- Children's Hospital Colorado, Aurora, CO
- Children’s National Medical Center, Washington, D.C.
- Children’s of Alabama, Birmingham, AL
- Phoenix Children’s Hospital, Phoenix AZ
- Seattle Children’s Hospital, Seattle, WA
- Stanford Children’s Health, Palo Alto, CA
- University of Texas Southwestern/Children’s Health, Dallas, TX
- Vanderbilt University Medical Center, Nashville, TN
- Yale Pediatric Neuromuscular Clinic, New Haven, CT
To establish best care, Cure SMA has partnered with SMA care centers throughout the U.S. to form the Cure SMA Care Center Network. Each care center is committed to improving care. These centers share information about how their center provides care to patients with SMA and they engage in reviewing and analyzing the data collected to continuously improve care and progress toward evidence driven care.
Interested in receiving care at a Care Center Network site? Please see the menus above to locate a center near you.
0
Cure SMA Care CentersAdult and Pediatric sites that provide FDA approved treatments for SMA.
0
States and Washington, D.C. with an SMA Care Center.0+
Patients with SMA receiving care across the SMA Care Center NetworkData as of 3/31/2023