Newborn Screening for SMA
Evidence shows that early diagnosis through newborn screening and early intervention with available treatments lead to better outcomes. This is especially true with spinal muscular atrophy (SMA), where early detection and timely administration of therapies can prevent the rapid and irreversible loss of motor function caused by the disease.
Quick Links
What Is Newborn Screening?
According to the Centers for Disease Control and Prevention (CDC), newborn screening identifies conditions that can affect a child’s long-term health or survival. Early detection, diagnosis, and intervention can prevent death or disability and enable children to reach their full potential. Each year, millions of babies in the U.S. are routinely screened—using a few drops of blood from the newborn’s heel—for certain genetic, endocrine, and metabolic disorders. They are also tested for hearing loss and critical congenital heart defects (CCHDs) prior to discharge from a hospital or birthing center.
In July 2018, the federal government added spinal muscular atrophy (SMA) to the Recommended Uniform Screening Panel (RUSP)—the list of suggested conditions that states should screen for within their statewide universal newborn screening programs. Since then, Cure SMA and its advocates have successfully encouraged the adoption of newborn SMA screening in all 50 states.
Frequently Asked Questions
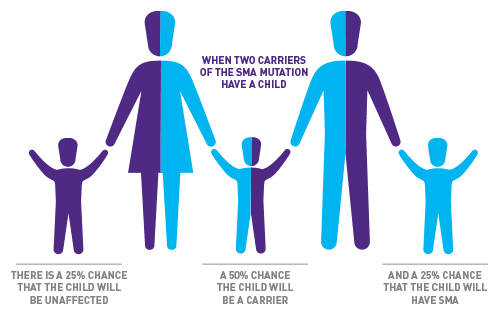
The figure above shows the chances that a healthy mother and father who are SMA carriers—each with one working SMN1 gene and one missing or faulty SMN1 gene—will have a child with SMA. In each pregnancy, the chance of these parents having a child with SMA is one in four, or 25%.
Newborn Screening Resources
We know this information may come as a surprise. We’re here to help. Cure SMA is nonprofit advocacy group that focuses specifically on SMA. You can find numerous resources for newly diagnosed families, and you can always contact us for information, guidance, or support. The below booklets offer a foundation for understanding SMA if you are a parent, patient, or healthcare provider learning about SMA for the first time.
Guide for Families: What You Need to Know and Do About an SMA Diagnosis
Guide for Healthcare Providers: What You Need to Know and Do About an SMA Diagnosis
To access and download all of our Cure SMA Care Series Booklets, click the button below
These booklets discuss topics from the genetics of SMA to nutrition, breathing basics, musculoskeletal system, mental health, and much more! Many of our booklets are offered in over ten languages.
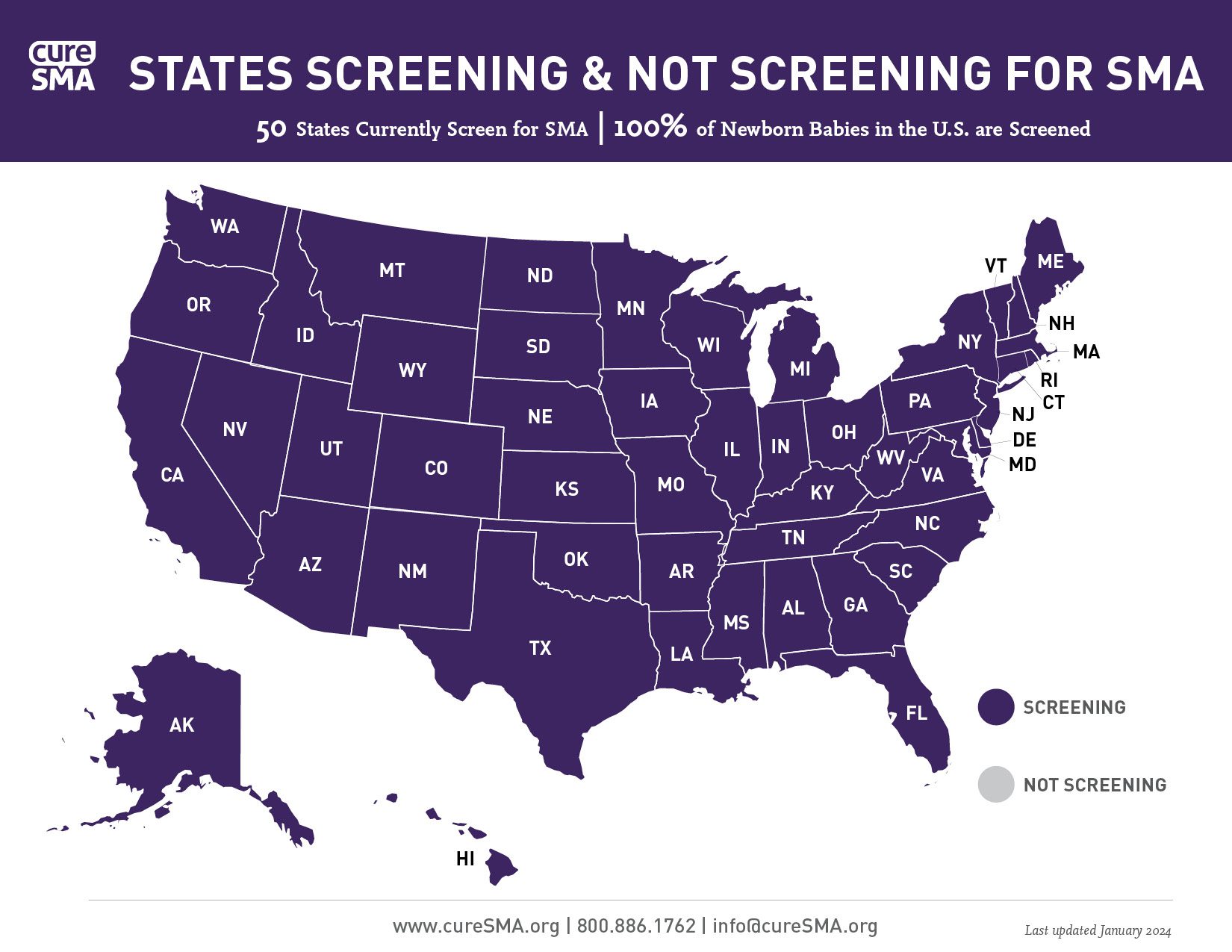
Is Your State Screening for SMA?
The status of newborn screening for SMA across the U.S. is shown on the following map — with all 50 states, plus Washington, D.C., screening for SMA. Within 6 years of SMA being added to the federally recommended list of diseases to screen for at birth, Cure SMA and its advocates have ensured that 100% of babies born in the U.S. are now screened for SMA at birth. To see our newborn screening state-specific fact sheets, please visit our Advocacy Overview page.
Download a PDF of the above newborn screening state program map, as of January 2, 2024.
Share Your Newborn Screening Experiences: Participate in a Survey
The SMA Care, Outcomes, and Reported Experiences (CORE) Survey aims to understand the diagnostic journey, time to treatment, and motor outcomes over time to improve care for newly diagnosed individuals, including individuals identified by newborn screening.
Primary caregivers of individuals with SMA that were diagnosed in the last 3 years and currently reside in the United States are eligible to participate.
If interested, please email the first and last name of you and the individual with SMA, as well as the year of SMA diagnosis to [email protected].