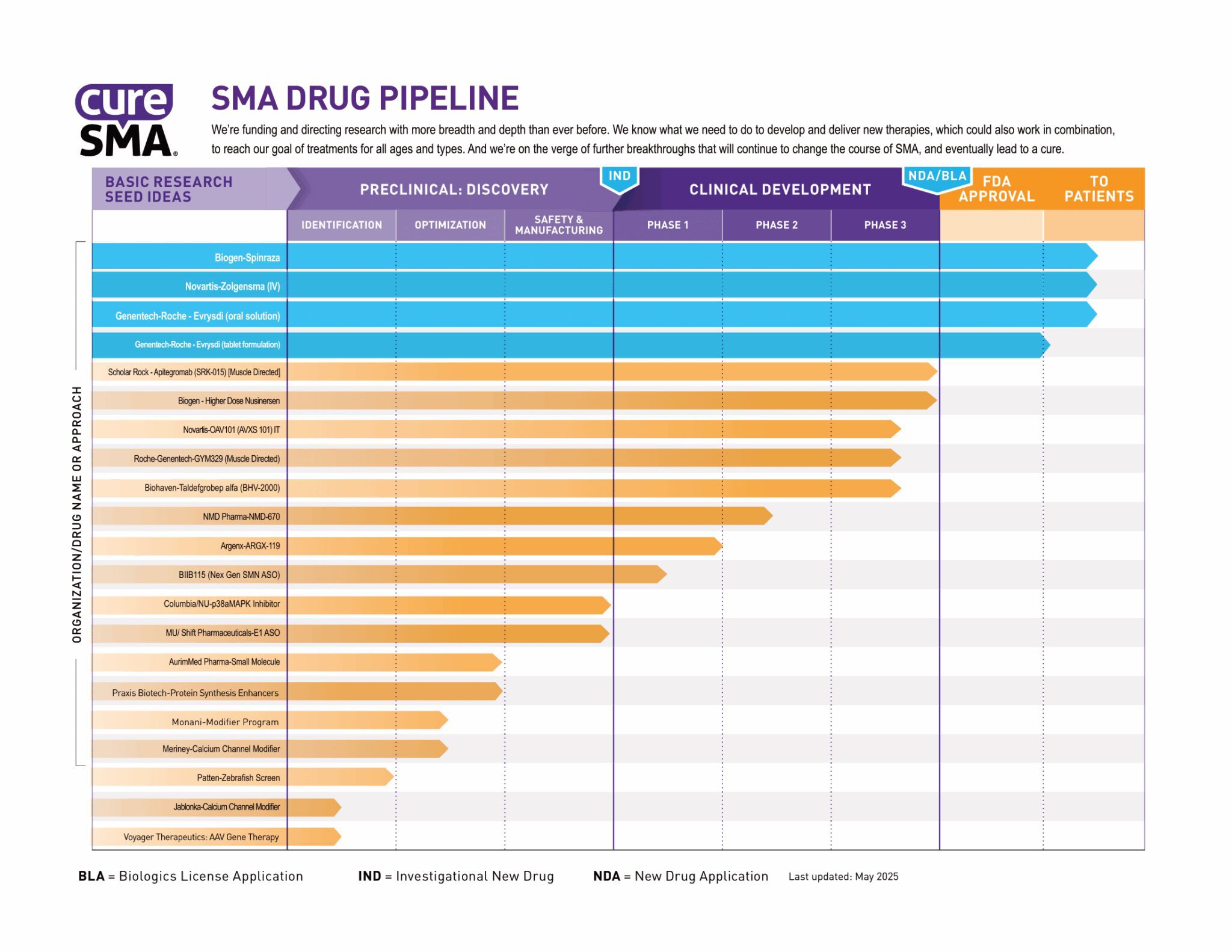
SMA Drug Pipeline
The SMA Drug Pipeline is how Cure SMA evaluates the success of our spinal muscular atrophy (SMA) research program. It identifies the major drug development programs and tracks their progress from basic research through FDA approval and beyond.
Key Measurements
The SMA Drug Pipeline measures the progress of our research in two ways. First, it tracks each program all the way to U.S. Food and Drug Administration (FDA) approval. Second, it tracks how those programs are spread among the different approaches to drug development.
Progress of Individual Drug Programs
Our research model funds drug programs at all stages of development. Our basic research program studies the biology and causes of SMA, often revealing new and more effective ways of making drugs. These basic research ideas are then converted into practical drug candidates through drug discovery. Finally, those drug candidates move through the clinical trial process. Our drug pipeline monitors each individual program as it moves through these stages.
Balance of Approaches to Drug Development
In order to find a cure for SMA, we know it's crucial to attack SMA from all sides. As with all scientific research, it's difficult to predict which SMA drug programs might be successful. By investing in a diversity of approaches, we maximize our chances for success. If one drug candidate or one approach fails, we have others to take its place.
The Cure SMA Drug Pipeline identifies several possible treatment targets:
Replacement or correction of the faulty SMN1 gene
Modulation of the low functioning SMN2 "back-up gene"
Muscle protection to prevent or restore the loss of muscle function in SMA
Neuroprotection of the motor neurons affected by loss of SMN protein
Newer approaches that identify additional systems and pathways affected by SMA
See Approaches to Drug Development for more details.
Approved Therapies for SMA
Several therapies have been approved for SMA. Zolgensma®, marketed by Novartis Gene Therapies®, replaces the faulty SMN1 gene. Evrysdi®, marketed by Genentech/Roche and Spinraza®, marketed by Biogen, modulate the SMN2 back-up gene. The Cure SMA Drug Pipeline continues to track these therapies as they are studied in ongoing clinical trials at different dosages and frequencies.
Approved drugs may also be studied in clinical trials that are designed to test “combination therapies.” Because of factors like age and severity of symptoms, SMA-affected individuals respond in a variety of ways to single therapies. A combination therapy is the use of two or more drugs to treat the same disease, either at the same time or at different stages in the disease, in hopes of achieving the best possible response to treatment. These drugs may have the same target, like the SMN2 back-up gene, or they may target different aspects of a disease. For example, one drug may target the SMN2 gene, while the second drug targets another protein that is important for muscle protection.