Table of Contents
What is Spinal Muscular Atrophy (SMA)
SMA or spinal muscular atrophy is caused by a mutation in the SMN1 gene (survival motor neuron gene 1). Typically, this gene produces a protein called Survival Motor Neuron (SMN), which is critical to the function of the nerves that control our muscles. When there are low levels of the SMN protein, as is the case in SMA, the nerve cells that control our muscles cannot function properly and eventually die, leading to muscle weakness that can be debilitating and sometimes fatal.
How Common is Spinal Muscular Atrophy (SMA)
SMA can be found in 1 in 11,000 births in the United States, and around 1 in 50 Americans are genetic carriers. SMA affects all races and genders.
Types of Spinal Muscular Atrophy (SMA)
Generally, we know that the number of SMN2 gene copies (the backup gene for SMN1) a person has impacts the severity of SMA. Having more copies of SMN2 is associated with less severe symptoms of SMA. Following the approval of the first disease-modifying treatment for SMA in 2016 (an SMN enhancing therapy) and the implementation of newborn screening for SMA in 2018, the historical classification of SMA does not adequately describe the new generation of infants and children with SMA treated early in life. In addition, more infants are being diagnosed and receive treatment before the onset of symptoms. With treatment, individuals are gaining more physical milestones and abilities than history would predict.
The SMA community of healthcare providers, people with SMA, and their families are having many conversations about how to best describe SMA disease in the era of disease-modifying SMA treatment. Characteristics that have risen to the top and that are generally used together include:
- Age of first treatment
- Impact of symptoms or the severity of symptoms
- SMN2 copy number (SMN2 is an important disease-modifying gene. Click here for more information)
- Maximum motor function achieved, for example, non-sitter, sitter, and walker
- Age of symptom onset
* The historical classifications of SMA before the availability of disease-modifying treatment were divided and characterized into five types of SMA: Type 0 SMA, Type 1 SMA, Type 2 SMA, Type 3 SMA, and Type 4 SMA. The different types were based on the highest physical milestone achieved and the age symptoms began.*

Causes of Spinal Muscular Atrophy (SMA)
Spinal muscular atrophy (SMA) is caused by a mutation in the survival motor neuron gene 1 (SMN1). A healthy person typically has two copies of the SMN1 gene. SMA occurs when both of an individual’s SMN1 copies have missing or mutated segments. Except in very rare cases, this happens when that individual has inherited two faulty copies of the gene—one from each parent. Individuals who have one faulty copy and one functioning copy of SMN1 are called carriers. Carriers do not have SMA, but they may pass the faulty gene on to their children. Approximately 1 in 50 people are a genetic carrier for SMA. Many times, people do not know they are carriers until they have a child born with SMA.
If both parents are carriers:
- 25% chance the baby is born with SMA
- 25% chance the baby is unaffected
- 50% chance the baby is a carrier
If only one parent is a carrier:
- 50% chance the baby is a carrier
- Very rare chance of a spontaneous genetic change to the SMN1 gene during sperm or egg production resulting in the baby having SMA
Carrier Testing
A DNA test is the only way to know if a person is a carrier of the SMA gene. The DNA test is a simple procedure, done through blood or saliva testing. In the general population, this test can detect about 95% of carriers. However, in African-American populations, detection is closer to 70%. This is because a difficult-to-detect mutation is seen more frequently in African-American populations than in other races.
The American College of Obstetricians and Gynecologists (ACOG) recommends that all women who are thinking about becoming pregnant, or who are already pregnant, be offered carrier screening for SMA and other genetic conditions. If the woman is a carrier, her partner can then be tested. In addition, individuals with a family history of SMA are often encouraged to have carrier screening. Deciding whether or not to undergo genetic testing is highly personal, and we strongly recommend discussing it with a physician or genetic counselor.
Symptoms of Spinal Muscular Atrophy (SMA)
SMA symptoms center around a loss of motor strength and/or not meeting certain motor development milestones on time. Symptoms can look different depending on the age of the person with SMA.
Child/Infant symptoms of SMA may present themselves as a difficulty or developmental delay in:
- Rolling over
- Sitting independently
- Head control
- Standing
- Walking
Older Children/Adults Symptoms of SMA may present themselves as:
- Struggling to keep up physically with their peer group
- Difficulty running
- Experiencing more fatigue than normal with activity
- Any mild motor weakness
While there can be many causes for developmental delays and motor weaknesses, SMA should be considered and discussed with your doctor.
Diagnosis
Diagnosing SMA can be done through a simple blood test that looks for a deletion of both copies of SMN1 gene. Around 95% of all SMA cases can be identified this way. The remaining 5% of SMA cases need to be diagnosed through a gene sequence analysis blood test because a heterozygous deletion on one SMN1 allele and a rare point mutation on the other SMN1 allele can not be identified through standard blood tests.
Prenatal testing is another way of diagnosing if a child has SMA in the womb. Families may pursue this type of testing if they are both carriers for SMA and after consulting their doctor. Two common prenatal SMA tests are:
- Amniocentesis, the most common form of prenatal testing, a very fine needle is inserted into the woman’s abdomen, and amniotic fluid is extracted. This fluid contains the baby’s DNA and can be tested for SMA. Amniocentesis can be performed after the 14th week of pregnancy.
- Chorionic Villus Sampling (CVS) can often be performed as early as the tenth week of pregnancy. Chorionic villi are small, fingerlike structures that form the placenta. Chorionic villi contain the baby’s DNA that can then be extracted and tested for SMA. CVS is associated with a risk of miscarriage.
SMA Newborn Screening
In 2018, SMA was added to the Recommended Uniform Screening Panel (RUSP), which is a list of suggested conditions that states should screen for within their statewide newborn screening programs. Now, thanks to the advocacy of Cure SMA and our advocates, all 50 states screen newborns for SMA. To screen a newborn, a blood sample is taken and tested for various genetic conditions. If a baby has a positive SMA screen, then other tests will be done to confirm the diagnosis.
Early diagnosis of SMA in newborns allows them to begin treatment earlier which has been shown to produce better disease outcomes. If your newborn has been screened and tested positive for SMA you can go to our Newborn Screening Q&A page for more information.

Acting quickly after a child is diagnosed with SMA is incredibly important because the earlier the treatment the better. If you are looking for support or help after an SMA diagnosis you can access our support programs, which are available to any SMA family in the United States. Most of the resources and programs we provide are at no cost to you.
Treatment for Spinal Muscular Atrophy (SMA)
SMA causes individuals to not produce the survival motor neuron (SMN) protein at high enough levels. With a deficit in the SMN protein, the motor neuron cells will shrink and eventually die. With this in mind, we can now discuss the treatment options for SMA.
One way of treating SMA is to increase the amount of survival motor neuron protein in the body. This is often called an “SMN-based” or “SMN-enhancing” approach.
All individuals with SMA have at least one, and often multiple, copies of a second gene called survival motor neuron gene 2 (SMN2) or the “SMA backup gene.” SMN2 also produces SMN protein but at a significantly lower quantity compared to the SMN1 gene.
Many SMN-enhancing treatments target the SMN2 gene, causing it to make more useable SMN protein. Other SMN-enhancing approaches work to replace the function of or repair the mutated SMN1 gene. Currently, all three FDA approved therapies work by increasing SMN protein. For more about current treatments, click here.
Outlook / Prognosis
Life expectancy before modern treatments was low for someone with SMA. However, with treatments now widely available today, life expectancy for someone living with SMA has improved greatly.
Quality of life has also increased since the development of these treatments and while SMA will still affect the nerves and muscles and therefore affect the basic functions of life such as walking, breathing, and movements, among others, SMA will not affect someone’s capacity to learn, think, and build relationships.
Living with Spinal Muscular Atrophy (SMA)
SMA will look different from person to person depending on a variety of factors such as the type of SMA they have, the age they began treatment, the individual’s age, and more. Since SMA affects the motor nerve cells and muscles, the symptoms can present themselves in many different ways. With such variation, you may find individuals with SMA who use motorized wheelchairs, others who utilize mobile flatbeds with breathing tubes, and individuals who are walking and, at first appearance, do not present any obvious symptoms.

For our resource guide on living with SMA for adults – Click here
For our resource guide on being a parent to a child living with SMA – Click here
Latest updates on Spinal Muscular Atrophy (SMA)
New medications, therapies, and treatments are always in the works for SMA. The image below will show you the major drugs and treatments in development and what stage of progress they are at. Some of these drug developments are supported by Cure SMA’s research funding.
The SMA Drug Pipeline measures the progress of Cure SMA’s research efforts in two ways. First, it tracks each program all the way to U.S. Food and Drug Administration (FDA) approval. Second, it tracks how those programs are spread among the different approaches to drug development.
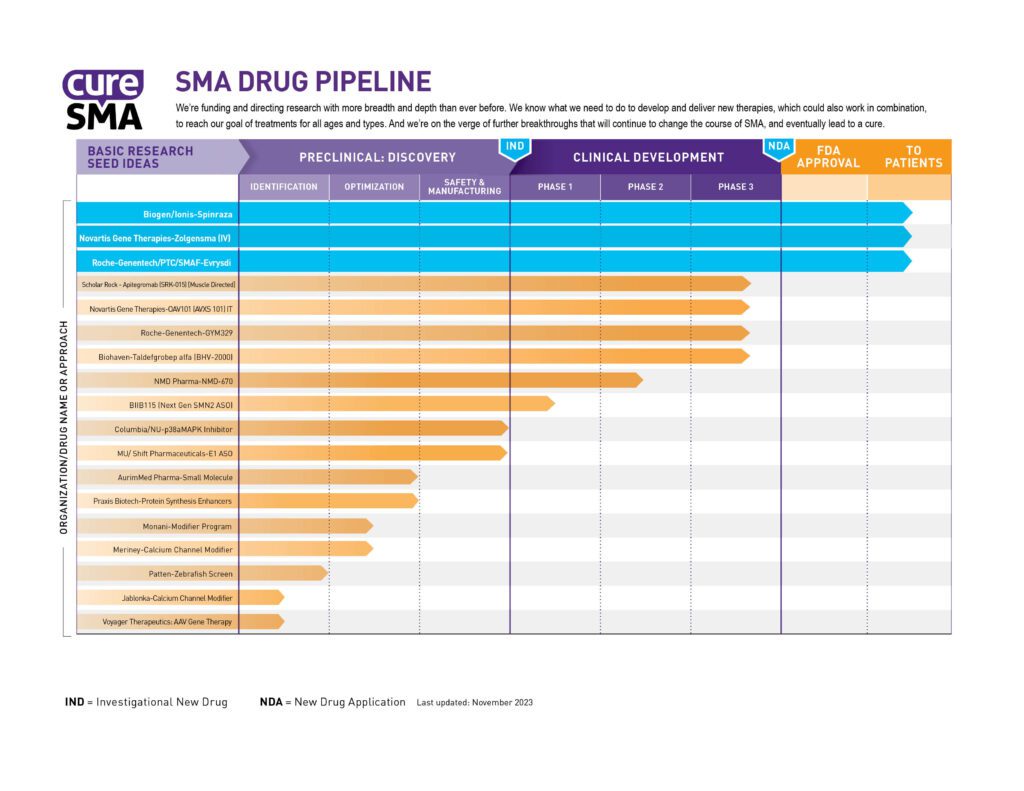
Besides SMA treatment, we are happy to share that SMA Newborn Screening is now taking place in all 50 U.S. states. This has been an initiative of Cure SMA for many years and finally came to completion in January 2024. SMA Newborn Screening will help to identify babies with SMA earlier so that they can benefit from starting treatment before symptoms appear. For more information on Newborn Screening, you can click here.
For the latest information on SMA from legislation to drug development, you can view our latest SMA news updates.
SMA Research
Basic SMA research helps in the development of new treatments and progress towards a cure. Research efforts focus on the causes and biology of SMA to help create more effective treatments going forward. For decades Cure SMA has invested in SMA research and since 2004, has invested over 15 million dollars toward basic SMA research. Thanks to our investments in research efforts:
- We helped develop multiple animal models of the disease. These models represent the full spectrum of disease severity, allowing us to better understand SMA and test new drug candidates
- We helped identify the role of the survival motor neuron (SMN) protein in SMA. This allows us to better understand the function it performs in the nerve cells that control our muscles. We now know that, because of the mutation in the SMN1 gene, individuals with SMA don’t produce this protein at high enough levels
- We helped discover the SMN2 gene. We now know that this “backup gene” can also perform the function of the SMN1 gene, meaning we have a target already in the body for drug therapies. The SMN2 gene has become the target of two FDA-approved SMA treatments
- We helped map and clone the survival motor neuron gene 1 (SMN1). We now know that SMA is typically caused by a mutation in this gene. The FDA has since approved an SMA treatment that replaces this faulty gene
For more information on basic SMA research, you can view Cure SMA’s published research by clicking here.
SMA Resources and Programs
There is a host of resources, support, and information to help you in your SMA journey. A list of some of the resources we and others offer are below:
- Care Series Booklets, which are publications giving information on specific topics to SMA
- Annual SMA Conference, the largest SMA conference in the world, and an opportunity for everyone in the SMA community to come together for resources, information, and community
- Treatment Site Locator, is a tool to help you find treatment centers that have the resources and specialties to care for those with SMA
- Cure SMA, is where you can access all of our resources and support programs
- Spinal Muscular Atrophy UK, is another website for those in the UK to get resources and support

Clinical Trials and Studies
SMA clinical trials are often complicated, difficult, and long, with only 1 in 10 drugs getting FDA approval that enter clinical development. Through the SMA Industry Collaboration, Cure SMA funds research to ensure that effective and safe treatments for SMA can progress through clinical trials quickly and gain approval from the FDA and international regulators. We also make sure that the drugs being developed meet the needs, priorities, and goals of the SMA community.
If you are interested in participating in a clinical trial for SMA research you can click here to view available clinical trials that are recruiting.
Common SMA Questions
Q – Is SMA Fatal?
A – SMA can affect life expectancy but this will vary widely based on the severity of symptoms, age of onset, and how early treatment began. With the development of modern SMA treatments, life expectancy has greatly improved, and new treatments are currently being developed.
Q – Is SMA Genetic?
A – Yes, SMA is a recessive genetic disorder. This means that if both parents are carriers and have a faulty SMN gene, there is a 25% chance of their child being born with SMA.
Q – How is SMA Diagnosed?
A – SMA is diagnosed through genetic testing which is done through a simple blood test. Other conditions besides SMA will also be tested because the symptoms of other diseases may be similar.
Q – Is SMA Common?
A – No, SMA is a rare disease with about 1 in 11,000 people being born with it in the United States.
For more commonly asked SMA questions and answers, click here.
Next Steps for Spinal Muscular Atrophy (SMA)
SMA can feel overwhelming and there are a lot of difficulties and challenges that come with an SMA diagnosis. But you are not alone and Cure SMA is here to support you. Below are some next steps and advice you should consider, but of course, all next steps should be discussed and planned with your doctor.
Next Steps and Advice:
- Talk with your doctor and create a plan going forward
- Acting quickly is crucial, especially in terms of treatment
- Cure SMA is here for you and can help. Contact us for information, guidance, free resources, and support



