SMA Clinical Trials
Learn more about the SMA clinical trial process, what it involves, why it matters, and how you can get involved.
Quick Links
What is a Clinical Trial?
A clinical trial is a type of study in which researchers test whether a new drug is safe and effective* for people.
Researchers must first study a new drug in the lab before they can test it in people. Data from lab studies may suggest that the new drug can safely help people with a certain disease. If so, researchers can apply for permission to study the drug in a clinical trial.
* Bolded words are terms that are commonly used in clinical trials. They are defined in the glossary below.
Interested in Exploring Clinical Trials
The SMA Registry and Clinical Trial Finder can be used to explore clinical trials and observational studies looking for participants.
If you have participated in a clinical trial in the past, you can also search for that trial and see if the results have been published.
If you are ready to begin your clinical trial journey click here.
Click here if you have questions about the SMA Registry and Clinical Trial Finder.
To explore all of Cure SMA’s new clinical trial resources, including Decoding Informed Consent, the Screening Visit Checklist, and the Travel Planners, click here.
How Do Clinical Trials Work?
There are four phases, or steps, in a clinical trial. A drug must pass through each phase of a clinical trial before moving to the next.
Researchers have specific goals for each clinical trial phase, and the number of people participating in each phase may differ. The numbers of participants given below are common for clinical trials that test drugs for an orphan disease like SMA.
Phase 1
Researchers test the drug’s safety and dosage in about 10 to 20 individuals. Sometimes the participants are healthy individuals. If the clinical trial is testing a drug for an orphan disease like SMA, some or all participants may have the disease.
Phase 2
Researchers continue to test the drug’s safety and effectiveness in 20 to 40 people. All participants have the disease being studied.
Phase 3
Researchers test the drug’s safety and effectiveness in 100 to 200 people. They often compare the new drug to a placebo or sham in this phase.
A drug that passes Phases 1-3 can be reviewed by the FDA for approval.
Phase 4
Researchers continue to monitor and evaluate the effects of the drug, even after a drug has been approved for the doctors to prescribe to the public.
For more information about clinical trials, visit our care series booklet, Learning About Clinical Trials, as well as our webinar SMA Clinical Trials: What You Need To Know.
Who Conducts Clinical Trials?
An organization that conducts a clinical trial is called a “sponsor.” A clinical trial sponsor may be a drug company, a non-profit group, or a government organization.
The sponsor funds the clinical trial and selects the trial site(s). Doctors’ offices, medical centers, and hospitals are examples of clinical trial sites. The clinical trial sponsor also gathers a team of medical and research professionals who will work with the clinical trial participants. You may hear this group of professionals referred to as the “study team.” The study team typically includes:
Principal Investigator
The principal investigator (PI) is usually a medical doctor who is often called the “study doctor.” The PI is responsible for directing the trial and the other doctors, nurses, and medical staff on the study team.
Clinical Research Coordinator
The clinical research coordinator (CRC) or “study coordinator” is often the first member of the study team you will meet when you are deciding whether to participate in a trial. The CRC manages the day-to-day activities of the trial.
Clinical Evaluator
A clinical evaluator (CE) is often a physical therapist who measures participants’ motor function, as well as other changes in their health, throughout the trial.
Cure SMA's Approach
The process of conducting clinical trials can be long, complicated, and difficult. On average, only one in ten drugs in clinical development get approved by the FDA. Through the SMA Industry Collaboration, we fund research to ensure that effective, safe treatments for SMA can progress through clinical trials quickly and gain approval from the FDA and international regulators. Our research also ensures these treatments address the unmet needs of the SMA community, and that the community’s priorities and goals are incorporated into the development, review, and approval of therapies.
Who Oversees Clinical Trials in the U.S.?
Three separate groups oversee clinical trials before they begin and while they are in progress. Each of these groups plays its own part in protecting the health and safety of clinical trial participants:
Food and Drug Administration (FDA)
Before a clinical trial begins, the Food and Drug Administration (FDA) approves a detailed plan known as a trial protocol. The trial protocol explains why and how researchers will conduct the trial. The FDA also makes sure that during the clinical trial, national rules that protect the rights of participants are followed. It does this in part by requiring that a special committee called an “Institutional Review Board (IRB)” monitors each clinical trial while it is being conducted.
Institutional Review Board (IRB)
Each clinical trial site is monitored by an Institutional Review Board (IRB). An IRB is an independent committee that includes people from medical, scientific, and non-scientific backgrounds. The IRB has many responsibilities, including reviewing all written information that is given to participants. The IRB also monitors active clinical trials and makes sure that the possible benefits of the trial outweigh the possible risks.
Data and Safety Monitoring Board (DSMB)
The Data and Safety Monitoring Board (DSMB) is an independent group that reviews the data researchers have collected during the clinical trial. The DSMB is also in charge of creating stopping rules. Stopping rules are reasons that the sponsor should stop the clinical trial early, such as if the new drug is causing serious side effects.
How Can I Learn About the Possible Benefits and Risks of Participating in a Clinical Trial?
When you have found a clinical trial that sounds interesting, email or call the local study team. The study team may ask you for information such as your/your child’s age, type of SMA, and treatment history. If this information matches with the trial’s eligibility criteria, the study team will schedule a screening visit. At the screening visit, you will meet with the study team to learn about the details of the clinical trial. Cure SMA’s Screening Visit Checklist can help you collect this information and store it in one place.
At or before your screening visit, the PI or another member of the study team will give you an informed consent form. The informed consent form explains the details of the trial, including its purpose, length of time, required procedures, key contacts, and any possible benefits and risks. If you decide to participate in the trial, you will give your consent by signing the informed consent form. This process is called the “informed consent process.” The informed consent process is in place to make sure you have all the information you need to make the best possible decision about whether to participate in a clinical trial. Learn more about informed consent by reviewing Cure SMA’s Decoding Informed Consent handout!
Not everyone who is interested in a clinical trial will have the chance to participate. This could be because you do not meet the eligibility requirements. It could also be that the trial has already enrolled the required number of participants. If you are not eligible for a particular trial, don’t give up! Regularly check the SMA Registry and Clinical Trial Finder to see if there is a new clinical trial that you/your child may be eligible for.
What Types of Treatments for SMA are Researchers Studying in Clinical Trials?
Researchers are exploring many ways to treat SMA.
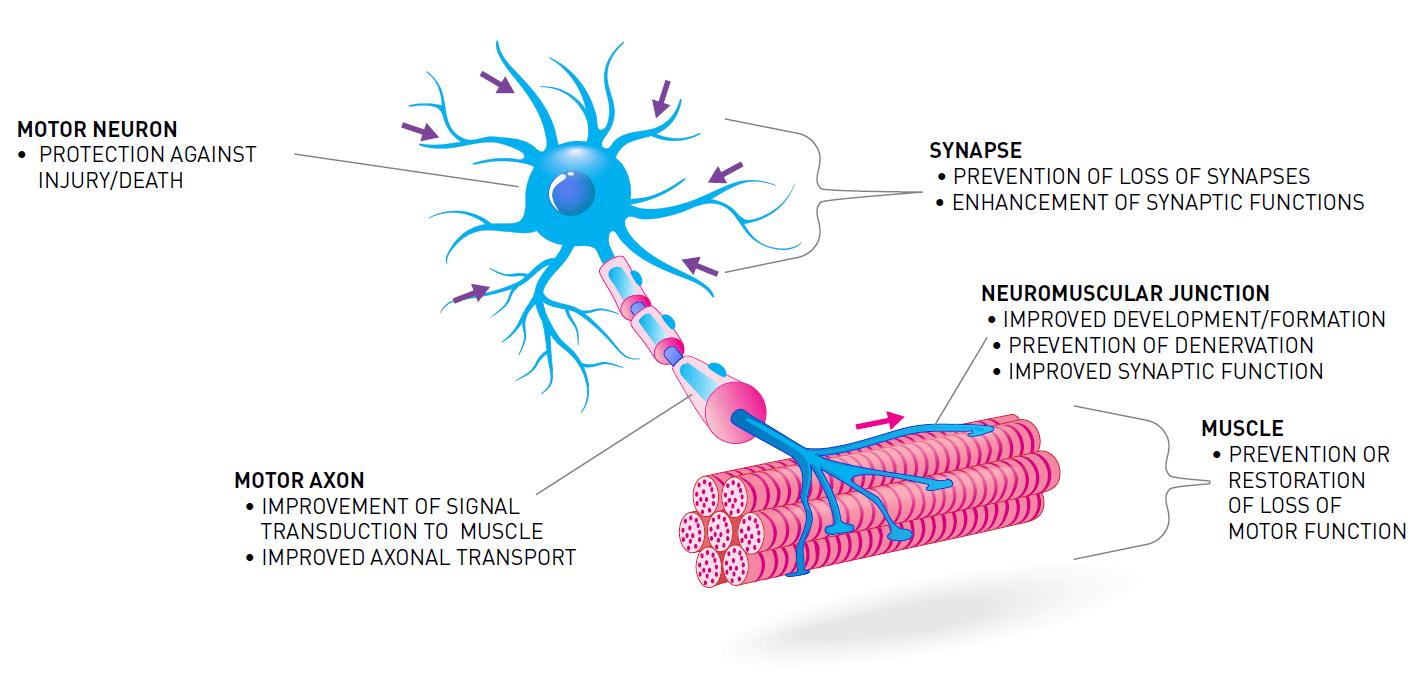
One way to treat SMA is to increase the amount of survival motor neuron protein (SMN) in the body. Treatments that increase the level of SMN protein are often called “SMN-dependent therapies." These include replacement or correction of the faulty survival motor neuron 1 gene (SMN1), and improving the output of the low-functioning survival motor neuron 2 gene (SMN2).
Increasing the amount of SMN protein in the body is not the only way to treat SMA. The loss of SMN protein also impacts other systems, pathways, and processes. Treatments aimed at these other targets are often called “SMN-independent therapies." SMN-independent therapies target different pathways that are involved in the loss of motor neurons and muscle function in SMA.
Clinical trials also provide an opportunity to compare outcomes from combination therapy with those from a single treatment. In combination therapy, two or more treatments that target SMA through different approaches are given together. Combination therapies for SMA may result in better outcomes than a single treatment because combination therapies target more than one aspect of the disease. Learn more about potential benefits and risks of combination therapy.
For more information on drugs that are being studied for the treatment of SMA, visit the SMA Drug Pipeline.
Traveling for Clinical Trials
Participating in a clinical trial for SMA may include traveling a long distance to get to the nearest trial site. Cure SMA’s travel planners will help you plan your trip to the clinical trial site. They are tailored to the unique needs of the SMA community.
Cure SMA has developed two travel planners: one for air travel and one for long-distance ground travel. These planners provide helpful reminders and space to organize important travel details.
Additional travel tips:
- Download the airline’s smartphone app. It may be easier to contact the airline through their app.
- The trial’s clinical research coordinator may arrange your air/ground travel for you and share insight on local accessible travel.
- Local cab companies may be able to provide accessible vehicles. Booking ahead of time with a cab company may be the best way to meet your unique needs.
- Accessible rideshare may be available, but it tends to be less reliable.
- The Transportation Security Administration (TSA) recommends arriving at the airport at least two hours before your flight. However, if you are flying with a wheelchair, aim to arrive three hours before your flight.
- Not all plane cargo doors are big enough to accommodate a wheelchair, especially smaller planes used for shorter or regional flights. After booking, call the airline to make sure the plane used for your flight will be able to accommodate your wheelchair.
Glossary of Key Terms
clinical evaluator (CE): a member of the study who is a physical therapist and monitors changes in the health and abilities of trial participants.
clinical research coordinator (CRC): a member of a clinical trial study team who manages the day-to-day activities of the trial.
clinical trial: a type of study in which researchers test whether a new drug is safe and effective for people.
combination therapy: when two or more treatments are given with the goal of achieving the best possible health outcomes. Often in combination therapy, each treatment targets a different aspect of the disease.
Data and Safety Monitoring Board (DSMB): an independent group that reviews the data researchers collect during a clinical trial. The DSMB also creates stopping rules for the trial.
dosage: the amount of a drug that is given to clinical trial participants.
effective: has the desired effect(s) on health or disease.
eligibility criteria: traits, like age or SMA type, that all participants in a study must share. Having research participants that all fit the same eligibility criteria makes it easier for researchers to evaluate the new drug’s safety and effectiveness.
Food and Drug Administration (FDA): the government organization in the U.S. that is responsible for protecting clinical trial participants’ health, safety, and privacy. The FDA is also responsible for evaluating clinical trial results and deciding whether new drugs are safe and effective for use in people.
informed consent: the process by which you are given all the information you need to make the best possible decision about whether clinical trial participation is right for you.
Institutional Review Board (IRB): an independent committee made up of doctors, researchers, and non-scientific people. Each IRB monitors a specific clinical trial site to protect participants health, safety, and privacy.
observational study: a research study in which researchers collect information from participants or look at data that was already collected without attempting to change the outcome with a new treatment or protocol (e.g., a registry).
orphan disease: a rare disease that affects less than 200,000 people in the U. S.
phase: a step in the clinical trial process for which there is a specific plan and goals. Each step must be completed before the new drug can move on to the next phase of testing.
placebo: an inactive substance that looks like the new drug but has no effect on health.
principal investigator (PI): a medical doctor who directs a clinical trial and its study team.
protocol: a detailed plan for a clinical trial or other research study.
screening visit: a meeting with the clinical trial study team in which you learn more about the trial and whether it is a good fit for you.
sham: a procedure that is the same as the one used to deliver a new drug but does not include the drug.
SMN-dependent therapy: an SMA treatment that works by increasing the amount of the SMN protein in the body.
SMN-independent therapy: an SMA treatment that works by doing something other than increasing the amount of the SMN protein in the body, like protecting muscle from shrinking and losing function.
sponsor: an organization that pays for and conducts a clinical trial. A sponsor may be a drug company, a non-profit group, or a government organization.
stopping rules: reasons that the sponsor should stop the clinical trial early, such as if the new drug is creating serious side effects.
study team: a group of doctors, physical therapists, and other health care workers who work together to conduct a clinical trial.
survival motor neuron 1 gene (SMN1): a gene that contains the instructions for the SMN protein, which is required for motor neuron health and function. Deletion or mutation of the SMN1 gene results in SMN protein deficiency. To be affected by SMA, an individual must inherit two faulty SMN1 genes, one from each parent.
survival motor neuron 2 gene (SMN2): the SMN protein’s “back-up” gene. Like the SMN1 gene, the SMN2 gene also contains the instructions for the SMN protein. However, much of the SMN protein made from the SMN2 gene is shortened and doesn’t function well.
survival motor neuron protein (SMN): a protein made from the instructions encoded in the SMN1 gene and the low-functioning SMN2 gene. The health and function of motor neurons is dependent on the SMN protein.
trial site: a doctor’s office, medical center, or hospital where a clinical trial is conducted.



