Recently, Cure SMA and colleagues at Novartis Gene Therapies published a manuscript titled, “Economic burden of spinal muscular atrophy: an analysis of claims data” in the Journal of Market Access & Health Policy. The purpose of the manuscript was to describe an analysis that was conducted measuring direct costs related to spinal muscular atrophy (SMA) management prior to the first U.S. Food and Drug Administration (FDA) approved therapy for SMA.
As new treatments continue to become available, the economic impact of SMA on patients and healthcare systems has been debated. To understand and quantify the impact of these therapies, it is important to have data on the economic burden of SMA prior to the availability of SMA treatments. These results will allow researchers to track cost-related changes over time as the use of new treatments increases. The economic burden of SMA was assessed by measuring the direct costs captured through inpatient and outpatient insurance claims from the Truven Health Analytics Marketscan Commercial Claims and Encounters and Medicare Supplemental databases. Inpatient claims covered costs incurred for a patient admitted into the hospital while outpatient claims cover costs for medical care that does not require a hospital admission.
The SMA patients included in the analysis were categorized as having infantile SMA (defined as having their first SMA diagnosis before the age of 1 year), child SMA (defined as having their first SMA diagnosis between the ages of 1 and 3 years), and juvenile SMA (defined as having their first SMA diagnosis between the ages of 3 and 18 years). All SMA patients were matched, one-to-one, to controls (i.e., non-SMA patients) based on age, gender, and geographic location to determine typical medical costs for the general population without SMA.
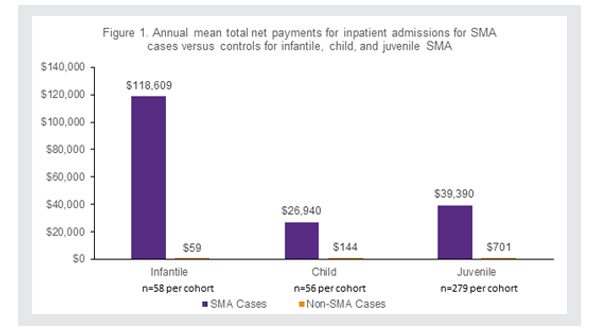
For all SMA patients, the inpatient and outpatient costs were higher, often more than 50 times higher, than their matched non-SMA patients (Figure 1).
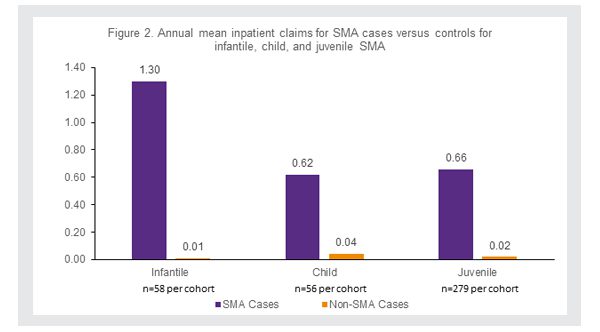
Additionally, the average number of inpatient claims per year were higher for SMA patients than their matched non-SMA patients, with respiratory illness the most common reason for an inpatient admission among all SMA patients (Figure 2).
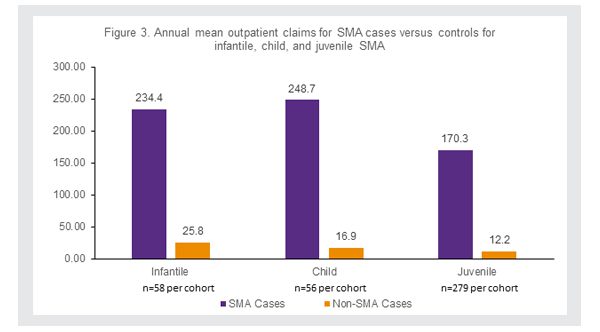
Similarly, the average number of outpatient claims per year were higher for SMA patients than their matched non-SMA patients, with a home health service the most common reason for an outpatient claim for all SMA patients (Figure 3).
Results of this analysis highlight that the economic burden of direct inpatient and outpatient costs are tremendous, averaging more than $100,000 per SMA patient per year. The availability of new therapies—and widespread newborn screening for SMA that leads to earlier diagnosis and treatment—has the potential to reduce the economic burden of SMA and significantly improve clinical outcomes and quality of life.
This study was financially supported by the Cure SMA Industry Collaboration. At the time financial support was provided, members of the Collaboration included Novartis Gene Therapies, Biogen, Genentech/Roche Pharmaceuticals, Novartis, Astellas, Cytokinetics, and Scholar Rock.
About the Cure SMA Industry Collaboration
The Cure SMA Industry Collaboration was established in 2016 to leverage the experience, expertise, and resources of pharmaceutical, biotechnology companies, and other nonprofit organizations involved in the development of spinal muscular atrophy (SMA) therapeutics to more effectively address a range of scientific, clinical, and regulatory challenges. It is currently comprised of our partners at Novartis Gene Therapies, Genentech/Roche Pharmaceuticals, Scholar Rock, Cytokinetics, and Biogen.



