Cure SMA’s primary focus is supporting care of the highest value to individuals with SMA. As a result of treatments approved by the U.S. Food and Drug Administration (FDA), as well as successful initiatives like the Cure SMA Care Center Network and SMA Clinical Data Registry, we have made significant progress enhancing the lives of people with SMA and their families. This week, Cure SMA shared a poster, titled “Cure SMA Care Center Network and SMA Clinical Data Registry with Electronic Medical Record Integration,” as part of the 2021 Virtual American Academy of Neurology (AAN) Annual Meeting.
In 2018, SMA standard of care guidelines were updated and approved based on consensus, due to limited evidence. With the advent of effective treatments and the implementation of newborn screening for SMA in 36 states to date, the need for an evidence-based standard of care for people with SMA is needed now more than ever. To create the evidence needed for this, Cure SMA established a Cure SMA Care Center Network and SMA clinical data registry to collect a cross section of real-world data about SMA care.
“Our goal is to ensure all people with SMA receive multidisciplinary and interdisciplinary standardized care and access to the FDA-approved treatments for SMA, regardless of where they live,” said Mary Schroth MD, FAAP, FCCP, Chief Medical Officer of Cure SMA. “We are excited about the progress of the Cure SMA Care Center Network and SMA Clinical Data Registry and look forward to continued growth with ongoing support from the SMA Community.”
As of today, the Cure SMA Care Center Network has 19 sites across the country, representing SMA care for both children and adults. Centers, a collection of neuromuscular clinics, were identified and invited to participate based on the following characteristics:
- Multidisciplinary and interdisciplinary standard of care model.
- Pediatric and adult care team partners or potential to achieve partnership.
- Diversity in geography and size.
- Capacity to provide FDA approved SMA treatments.
- Ability to integrate with electronic medical records.
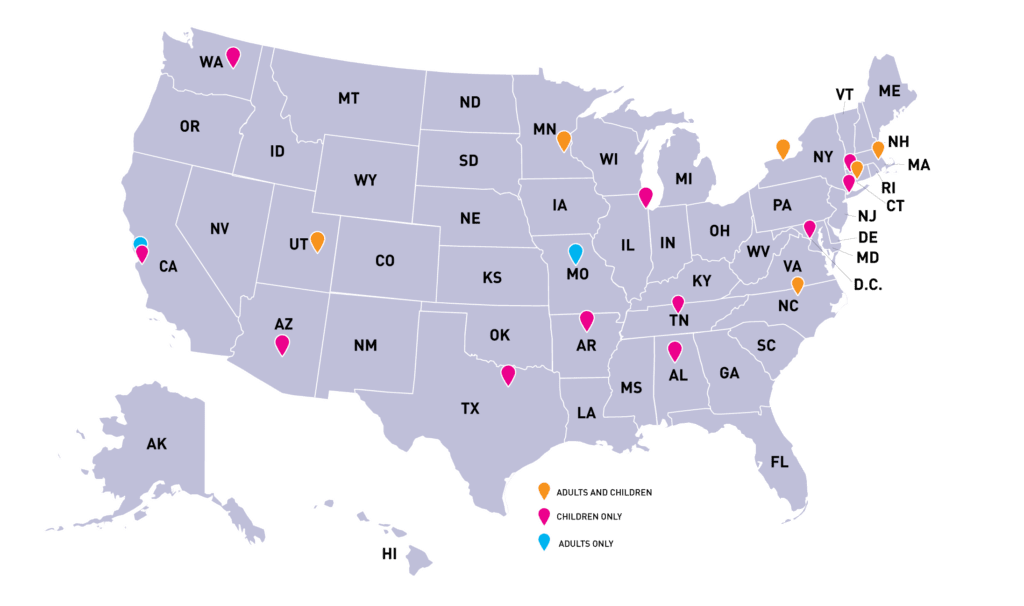
Centers in the Network provide multidisciplinary care for people with SMA and contribute consented SMA patient data securely and electronically transferred from the electronic medical records to the SMA Clinical Data Registry. This clinical information is real-world evidence that will be used to guide best care and create evidence-based standards of care for SMA. And by utilizing a united registry to collect data, healthcare providers will be able to collaborate and receive benchmarking information about care at their Center. The SMA Clinical Data Registry continues to grow in partnership with the Cure SMA Care Center Network. In the poster, the Registry is reported to include 494 patients.

Dr. Schroth and her team conclude: “The development of the Cure SMA Care Center Network and electronic medical record data transfer to the SMA Clinical Data Registry is feasible, scalable, and the best solution to create evidence-based care guidelines and to understand the impact of SMA gene-enhancing treatments.”
Acknowledgements: The Cure SMA Care Center Network is supported in part by a grant from the Oscar G. and Elsa S. Mayer Family Foundation and an endowment from Bill and Susan Orr and the Tyler William Orr Memorial Fund. Cure SMA has partnered with Prometheus Research for the SMA Clinical Data Registry platform.



