Cure SMA is pleased to announce the participation of scientific leadership in the 2022 Muscular Dystrophy Association (MDA) Clinical & Scientific Conference (March 13–16, 2022), the American Academy of Neurology (AAN) 2022 Annual Meeting (April 2–7, 2022, and the upcoming Cure SMA Research & Clinical Care Meeting (June 15–17, 2022).
Our poster presentations feature data from research initiatives launched by the Cure SMA Industry Collaboration (SMA-IC). The SMA-IC is a partnership that brings together pharmaceutical companies, Cure SMA, and other patient advocacy groups to share information, ideas, and data. The collaboration works to address scientific, clinical, and regulatory topics that are critical to advancing SMA drug development and will benefit the broader SMA community. The ongoing goals of the SMA-IC include:
- Distribution of a benefit-risk survey to assess the specific risks SMA-affected individuals and families would be willing to accept in exchange for certain treatment benefits
- Development and refinement of outcome measures
- Distribution of a survey to acquire demographic information from individuals and families affected by SMA
- Organization of focus groups to understand and identify disparities and gaps in SMA care, treatment, and clinical trial research access
- Evaluation of five years of data captured via the annual Community Update Survey to better understand changes in the SMA disease experience and patient needs
- Development of educational materials to highlight the impact of copy number and disease status at time of treatment on health outcomes for affected individuals
Below is a re-cap of the posters we have presented this spring and will be sharing at the Cure SMA conference in June.
Assessing the Impact of COVID-19 in Health Care Providers Treating Spinal Muscular Atrophy (SMA)
Between November 2020 and March 2021, Cure SMA conducted a survey designed to understand how the COVID-19 pandemic impacted SMA-patient care and clinical trial management in the US. The survey was distributed to health care providers at known SMA treatment and clinical trial sites. It was designed to determine how often and why appointments for either SMA-related care and services (e.g., physical therapy) or clinical trial participation were cancelled or delayed. We also asked providers at sponsor-initiated clinical trial sites what strategies they had come up with to avoid trial disruption during the pandemic.
Providers reported that at the height of the pandemic, patients often cancelled/delayed appointments for SMA-related care and services to avoid possible exposure to COVID-19. During this period, clinics also frequently cancelled/delayed appointments for drug treatment, care, and services. However, by the end of the study, appointment cancellations/delays by both patients and clinics had become less common. (Table 1)
SMA clinical trials were similarly affected. Of the clinical trial site providers who responded to the survey, 73.1% reported an increase in cancelled or rescheduled visits by trial participants. In addition, providers reported delays in trial visits due to either clinical staff exposure to (96.2%) or diagnosis with COVID-19 (96.2%). Several strategies were reportedly used by sites to minimize the effect of the pandemic on SMA clinical trials. When asked to rate the overall helpfulness of these strategies, providers rated telemedicine and prioritization of assessments as most helpful (Figure 1).
The results of this survey provide insight into the effect of the COVID-19 pandemic on the SMA community by helping us both understand the reasons behind appointment cancellations/delays, as well as identify some possible strategies to avoid them.Given the life-altering and life-saving impact, the administration of FDA-approved treatments must remain a . However, scheduling of SMA-related care, service, and clinical trial appointments are also of importance. Cure SMA encourages health care provider teams to collaborate with SMA-affected individuals and families to develop and implement strategies that address and mitigate ongoing COVID-19 exposure concerns while supporting patient outcomes and trial participation.
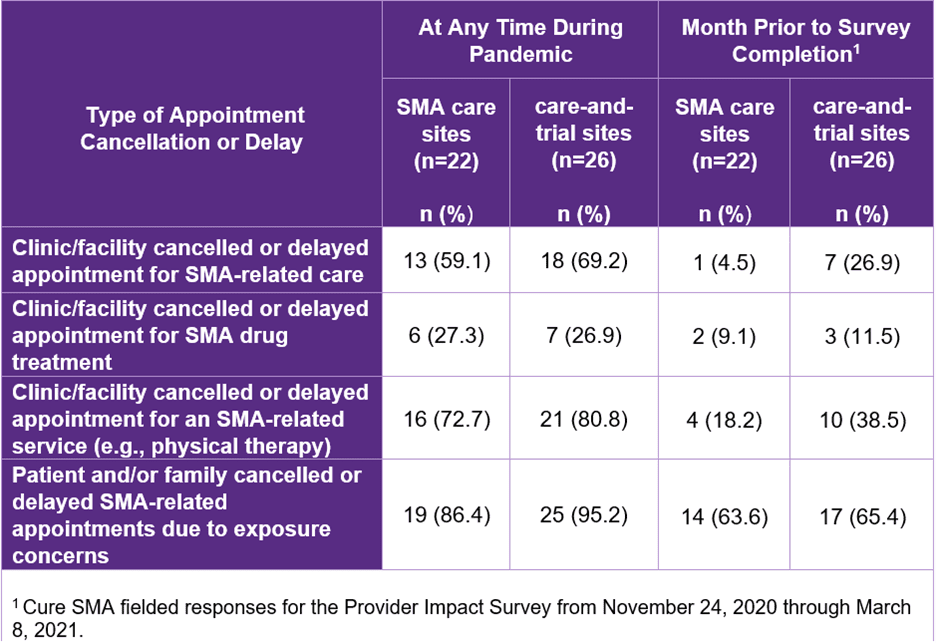
Table 1: Types of Appointment Cancellations/Delays Across Sites During Pandemic
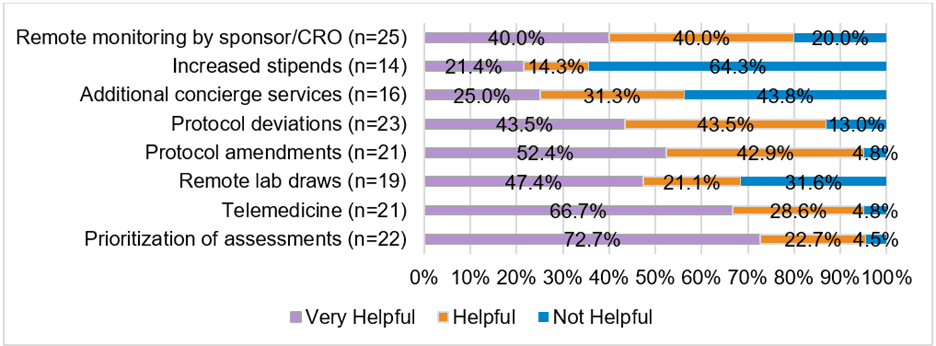
Figure 1: Strategies for Mitigating Impact on Clinical Studies. Respondents at clinical trial centers rated the helpfulness of strategies used to mitigate the pandemic’s impact on clinical trial conduct.
Assessment of Transition Plan Implementation and Provider Engagement Among Adults Affected by Spinal Muscular Atrophy (SMA)
The transition from pediatric to adult care is a major milestone for individuals living with SMA and their caregivers. A formal transition process helps SMA-affected patients and families prepare for the eventual shift from pediatric to adult care. The process should include a transition-readiness assessment to determine the affected youth’s decision-making capacity, and the creation of a formal transition plan1-3.
In August 2021, Cure SMA conducted a survey to learn from our community members about their experiences with the care transition process. The survey was distributed to SMA-affected adults ages 18 to 29 whose contact information was obtained from the Cure SMA membership database. One hundred and thirty-nine individuals responded to the survey. The results revealed that 11.5% of these respondents had not initiated the transition process, 12.2% were preparing to transition, 1.4% were preparing to transition and had initiated consults, 22.3% had partially transitioned, and 52.5% had fully transitioned to adult care (Figure 2).
Most respondents indicated that their pediatric care provider had not included one or more elements of an optimal transition planning process (e.g., transition readiness assessment, transition plan, pre-transition discussions and decision making, goals discussed, pre-transition consults and self-care guidance) (Figure 3). Of the respondents who were preparing to transition or that had partially/fully completed the transition process, 51.2% reported that their pediatric providers had not assessed their transition readiness. However, 43.9% of respondents had discussed the transition process and/or the development of a transition plan with their pediatric provider; yet among these respondents, just 16.7% had received a copy of the transition plan. Furthermore, 27.6% of the respondents who were preparing to transition or that had partially/fully transitioned to adult care reported “self-transitioning” without the assistance of pediatric providers.
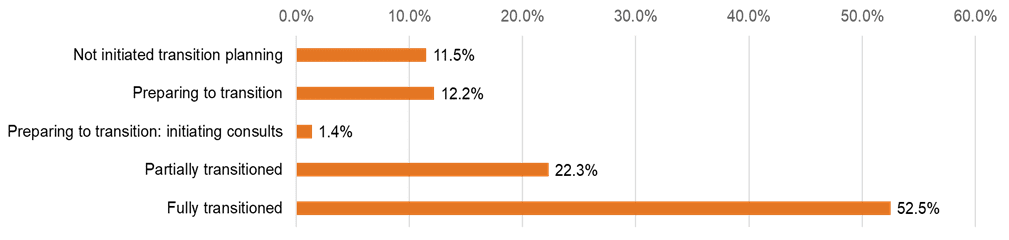
Figure 2: Transition Status of Survey Respondents
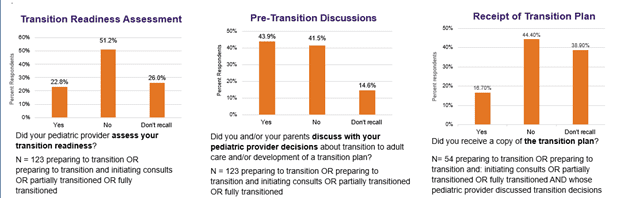
Figure 3: Process to Devise and Implement Transition Plan
Respondents were also asked how pediatric providers could improve the transition process and further support individuals and families affected by SMA as they transition to adult care. Top suggestions included, “have discussions about differences between pediatric and adult care” (92.8%), “provide access to comprehensive overviews of medical history” (90.6%), and “encourage greater involvement of adolescents and families in the transition planning process” (89.9%) (Table 2).

Table 2: Respondent Suggestions for Improving the Transition Process
We learned from this survey that the type and amount of support pediatric providers give to SMA-affected individuals during their transition between pediatric and adult care varies widely. Given the importance of continuity of treatment and care for the SMA population, preparation for the transition to adult care should remain a priority for pediatric providers. We encourage health care provider teams to collaborate with SMA-affected individuals and families to develop and implement transition plans. These plans should include goals and objectives that may mitigate barriers, encourage the management of self-care, and optimize patient experiences. Providers should also consider opportunities to address disparities in access to treatment, care, and management among SMA-affected individuals transitioning to adult health care services.
Predictors of Telemedicine Exposure, Comfort, and Perceived Effectiveness in the SMA Community
The use of telemedicine, which uses telecommunications technology like video conferencing to enable remote health care, is increasingly being utilized in health care diagnosis and delivery. This increase was accelerated by the COVID-19 pandemic and associated changes to insurance reimbursement policies.4-6 Telemedicine holds special interest for neuromuscular disease communities because of its potential to reduce mobility challenges associated with accessing health care and to increase access to those who may not live near an SMA-health care specialist.7 However, its use in neuromuscular settings also poses unique challenges. Recognizing the potential that telemedicine holds for the SMA community, Cure SMA sought insight into patient attitudes toward telemedicine by including questions on these topics in its 2021 Community Update Survey.
Four hundred and sixty-eight individuals affected by SMA or their caregivers responded to questions about telemedicine. Approximately four-fifths of these respondents indicated they had used telemedicine at least once. Most respondents were either comfortable or very comfortable with telemedicine, with self-completed surveys reflecting higher comfort levels. A relatively small proportion of respondents viewed telemedicine as very effective for managing and treating their SMA, although most thought it was at least moderately effective. (Table 3)
Controlling for key demographic and health factors, the likelihood of using telemedicine for SMA-related care varied based on gender, income, SMA type, SMA drug treatment status, and history of mental illness. Comfort with the technology varied based on whether the respondent was the affected individual or a caregiver, mobility status, SMA drug treatment status, whether the person had an in-person doctor’s visit in the last year, and history of mental illness. Perceived effectiveness of telemedicine varied based on mobility status, SMA drug treatment status, whether the person had an in-person doctor’s visit in the last year, and history of mental illness.
From these results, we learned that many in our community are comfortable with telemedicine and view it as an effective healthcare tool. However, attitudes toward telemedicine varied from person to person depending on a wide variety of factors. These insights will be helpful to providers who are considering utilizing telemedicine in the care of SMA-affected individuals.
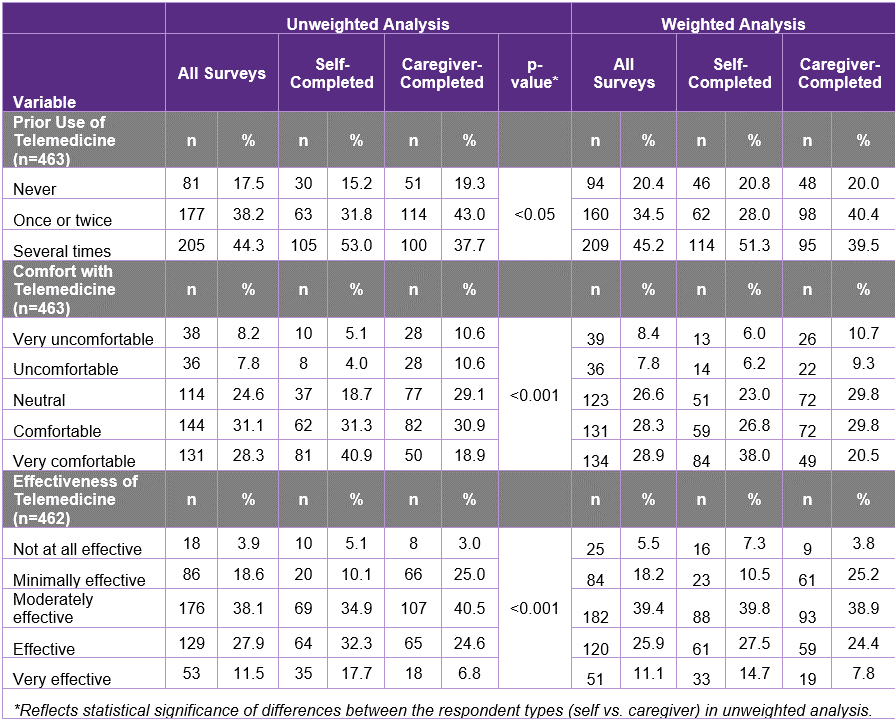
Table 3: Prior Use of and Attitudes about Telemedicine
Understanding the Caregiver Experience. Results from the Cure SMA Caregiver Experience Survey
In December 2021, Cure SMA launched an online survey to broaden our understanding of the caregiver experience and the burden of caring for a loved one with SMA. We also wanted to learn about the direct and indirect costs of providing care. Information about caregiver experiences was collected through questions about employment, respite services, work productivity, and quality-of-life measures. Here we present an overview of the initial findings from the survey.
Caregivers from the Cure SMA membership database received an email link to complete an online survey between December 2‒13, 2021. Two hundred and eighty-three caregivers completed the survey. The majority of family caregivers were female (83.3%), between ages 30‒39 (40.3%), White (85.2%), non-Hispanic (87.6%), and a current caregiver of an individual with SMA (95.9%) (Table 4). Of current caregivers, 62.7% were employed part- or full-time and reported providing an average of 63.7 hours of care per week to an individual with SMA. Among family caregivers who were ever employed while caring for an individual with SMA (n=211), 94.8% reported their employment being impacted by at least one of the following factors: lost job benefits, received a warning about work performance, leaving work entirely or cutting back hours, and turning down a promotion.
The impact of caregiving on work performance was assessed with the Caregiver Work Limitations Questionnaire (Caregiver WLQ). The Caregiver WLQ is a 22-item questionnaire that asks employed caregivers to rate the amount of time difficulty was experienced handling certain parts of their job while also providing care for someone who is ill or disabled. Of the 283 respondents, 148 completed the WLQ. Based on the results from the Caregiver WLQ, we determined that respondents who were caring for someone affected by SMA while employed were approximately 6.7% (SD 4.3%) less productive at their job than they would be if they did not have caregiver responsibilities. In addition, 233 (82.3%) of caregivers indicated they experienced financial strain as a result of caring for an individual with SMA.
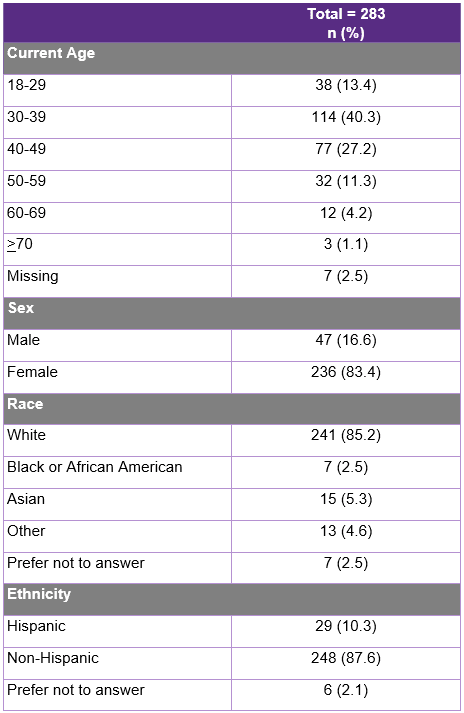
Table 4: Demographics of Survey Respondents
The results of this survey underscore the many areas, including employment, work performance, and finances that may be impacted when a family member (i.e., spouse/parent/sibling) provides care for an individual with SMA. Although the SMA community has made huge strides in treatment and care for those with SMA, it is important to assess and support the unmet needs of family members who serve as caregivers.
Why Have You Been Treated with More Than One SMA Therapy? Data from the 2021 Community Update Survey
Since 2017, Cure SMA has hosted an annual Community Update Survey to collect current experiences related to living with SMA and to track changes over time throughout the community. In response to increased interest in therapeutic approaches that combine the three FDA-approved SMA treatments, we used the 2021 Community Update Survey to gather information about patient opinions and perceptions that lead to participating in combination therapy.
The final data set from the 2021 Community Update Survey is composed of 633 completed surveys representing 615 unique SMA-affected individuals. For this analysis, residents of foreign countries (n=80), those with an unknown treatment status (n=90), those who had not been treated with an SMA therapy (n=67), and those with duplicated surveys completed (n=10) were excluded. The final sample size included 386 surveys completed by 154 (39.9%) SMA-affected adults and 232 (60.1%) caregivers of affected adults and children.
One hundred and forty-eight (38.3%) respondents indicated they have been treated with more than one FDA-approved therapy, and of these individuals, 11 (7.4%) have been treated with all three FDA-approved therapies. In addition, of those treated with combination therapy, 100 (67.6%) indicated they were treated sequentially; i.e., these individuals had discontinued either nusinersen or risdiplam prior to starting another FDA-approved treatment. The median (range) age of those treated with more than one treatment, either in combination or sequentially was 13 (0-71) years, with 25% of the individuals < 3 years of age. The self- or caregiver-reported SMA type of those treated with more than one SMA therapy was 1 (0.7%), 59 (39.9%), 52 (35.1%), 34 (23.0%), and 2 (1.4%) for Type 0, Type I, Type II, Type III, and unknown type, respectively. When asked the rationale for pursuing multiple SMN-dependent therapies, 37.5% of respondents indicated they were not gaining function as expected, 8.3% indicated they were losing motor function, and 80% indicated they wanted all possible SMA treatments.
In summary, we learned from the 2021 Community Update Survey that over one-third of SMA-affected respondents have been treated by more than one FDA-approved therapy, whereas a small percentage have been treated by all three. Most of those receiving combination therapy received one therapy at a time, and the majority had SMA Types I-III. The most common reasons for wanting to pursue combination therapy were to gain more function and to try all possible treatment options.
These data help to identify caregivers’ and patients’ rationales for pursuing combination therapy. The results of this survey emphasize a call to action to recognize that combination treatment is occurring and we as a research and clinical community have an obligation to collect more data to analyze the safety and effectiveness of combination therapy.
About the Cure SMA Industry Collaboration
The Cure SMA Industry Collaboration (SMA-IC) was established in 2016 to leverage the experience, expertise, and resources of pharmaceutical and biotechnology companies, as well as other nonprofit organizations involved in the development of spinal muscular atrophy (SMA) therapeutics to more effectively address a range of scientific, clinical, and regulatory challenges. It is currently comprised of our partners at Biogen, Genentech/Roche Pharmaceuticals, Scholar Rock, Novartis Gene Therapies, Biohaven Pharmaceuticals, Epirium Bio, and SMA Europe.
Funding for this research was provided by members of the 2020 and 2021 SMA-IC which included, Genentech/Roche Pharmaceuticals, Novartis Gene Therapies, Biogen, Cytokinetics, and Scholar Rock.
References
- Brown LW, Camfield P, Capers M, et al. The neurologist’s role in supporting transition to adult health care: A consensus statement. Neurology. 2016;87(8):835-840.
- Lebrun-Harris LA, McManus MA, Ilango SM, et al. Transition Planning Among US Youth with and without Special Health Care Needs. Pediatrics. 2018;142(4).
- McManus MA, Pollack LR, Cooley WC, et al. Current status of transition preparation among youth with special needs in the United States. Pediatrics. 2013;131(6):1090-1097.
- Friedman AB, Gervasi S, Song H, et al. Telemedicine catches on: Changes in the utilization of telemedicine services during the COVID-19 pandemic. Am J Manag Care. 2022;28(1):e1-e6.
- Koonin LM, Hoots B, Tsang CA, et al. Trends in the use of telehealth during the emergence of the COVID-19 pandemic – United States, January-March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(43):1595-1599.
- Volk J PD, O’Brien M, Goe CL. States’ Actions to Expand Telemedicine Access During COVID-19 and Future Policy Considerations. 2021.
- Wechsler LR, Tsao JW, Levine SR, et al. Teleneurology applications: Report of the Telemedicine Work Group of the American Academy of Neurology. Neurology. 2013;80(7):670-676.



