As treatment options increase for adolescents and young adults living with SMA, we need tools to measure the effectiveness of these treatments from the patient’s perspective. Dr. Chad Heatwole and his team at the University of Rochester have developed the Spinal Muscular Atrophy Health Index (SMA-HI) for this purpose.1 The SMA-HI is an SMA-specific survey tool that measures the overall burden of the disease by assessing the full range of SMA symptoms as well as other quality-of-life factors.
In this study, we wanted to determine whether the SMA-HI is effective for measuring patient-reported disease burden in SMA-affected adolescents and young adults. We also wanted to know if the SMA-HI could be used to determine the specific symptomatic areas that most affect quality of life for these populations. Finally, we wanted to provide a baseline study for researchers who consider using the SMA-HI to measure treatment effects in future SMA clinical trials involving adolescents and young adults. The results of this survey, “Assessing Perspectives of Disease Burden and Clinically Meaningful Changes Using the Spinal Muscular Atrophy Health Index in Adolescents and Young Adults” were published in the peer-reviewed journal, Muscle and Nerve.2
How We Did It
Using our Cure SMA membership database, we identified 412 individuals living with SMA between the ages of 12-25 years. We invited these individuals to participate in the SMA-HI survey through email blasts, social media posts, and research recruitment newsletters. The online survey was available from November 26, 2018, through January 21, 2019.
The survey contained demographic questions (e.g., age, SMA type), the SMA-HI, and four additional open-ended questions about education and socialization. Results from the latter four questions have been published in a separate manuscript.3 The SMA-HI consists of 107 questions divided into 15 subscales such as “gastrointestinal function” and “fatigue.” Each question asks the respondent, “How much does the following impact your life now?” Participants can choose between seven responses that range from “I don’t experience this” to “It affects me severely.” After a completed survey is submitted, the respondent’s answers are converted to numerical values, and the values within each subscale are added together to give a subtotal for that subscale. Statistical analysis was used to identify significant differences between scores categorized by age group, SMA type, and motor function. For a full description of scoring and analysis, please refer to published manuscript.2
What We Found
Eighty-eight persons living with SMA or their parent or caregiver completed the survey; 39 respondents were adolescents, and 48 were young adults. The majority of respondents had Type II or Type III SMA. At the time of the survey, 32% of respondents were non-independent sitters, 51% were independent sitter/non-independent walkers, and 17% were independent walkers.
When we compared results by age group, we found that the mean (average) total SMA-HI score did not differ significantly between adolescent and young adult respondent groups. However, young adults reported significantly higher subscale scores than did adolescents in hand and finger strength, swallowing function, sleep, and mobility and ambulation. It is interesting to note that one subscale that did not vary much between age or SMA type was hip, thigh, and knee function. This may be because this area of the body is affected early in the course of the disease. (See Table 2, which originally appeared in the publication, “Assessing Perspectives of Disease Burden and Clinically Meaningful Changes Using the Spinal Muscular Atrophy Health Index in Adolescents and Young Adults”.2)
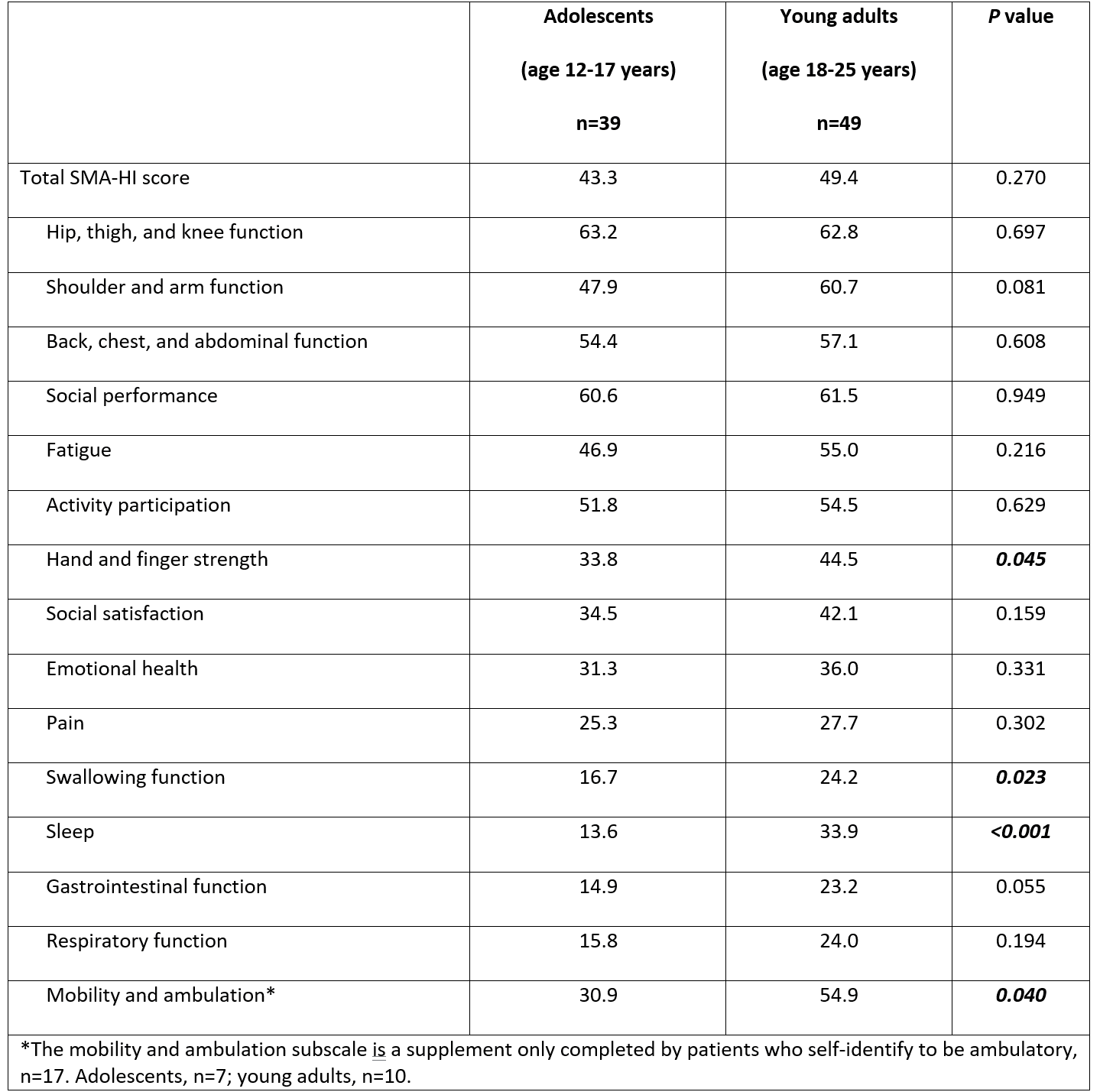
Table 1: Mean SMA-HI subscale scores by age group2
Comparing results by SMA type revealed that respondents living with SMA Types I and II experienced a significantly higher burden of disease in several subcategories, as well as a higher overall burden of disease, than did those living with Type III SMA. Likewise, higher scores correlated with greater limits on motor function.
Why It Matters
In this study, we found that the SMA-HI is an effective survey tool for measuring the degree and nature of disease burden for adolescents and young adults living with SMA. The findings also revealed that burden of disease was greater for young adults than adolescent respondents in the areas of hand and finger strength, swallowing function, sleep, and mobility and ambulation. Total disease burden was generally greater for those with more severe types of SMA, as well as for those with more limited motor function.
We also compared our results to those obtained by researchers at the University of Rochester. As part of their efforts to develop the SMA-HI survey, they queried 359 SMA-affected adults between 18 and 81 years of age, with a mean age of 42 years.1 We found that adolescents and young adults who completed this survey generally reported lower subscale and total SMA-HI scores than did the adult population in the previous study, which suggests that disease burden may increase with age. However, scores in areas such as emotional health, social satisfaction, and pain varied less between age groups. Together, these findings support the use of the SMA-HI with adolescent and young adult populations living with SMA.
Funding for this research was provided by members of the 2018 Cure SMA Industry Collaboration, which included, Astellas, Biogen, Genentech/Roche, Cytokinetics, Novartis, Novartis Gene Therapies, and Scholar Rock.
About the Cure SMA Industry Collaboration

The Cure SMA Industry Collaboration (SMA-IC) was established in 2016 to leverage the experience, expertise, and resources of pharmaceutical and biotechnology companies, as well as other nonprofit organizations involved in the development of SMA therapeutics, to address a range of scientific, clinical, and regulatory challenges more effectively. It is currently comprised of our partners at Novartis Gene Therapies, Biogen, Genentech/Roche Pharmaceuticals, Scholar Rock, and SMA Europe.
References
- Zizzi CE, Luebbe E, Mongiovi P, et al. The Spinal Muscular Atrophy Health Index: A novel outcome for measuring how a patient feels and functions. Muscle Nerve. 2021;63(6):837-844.
- Mazzella A, Cruz R, Belter L, et al. Assessing perspectives of disease burden and clinically meaningful changes using the Spinal Muscular Atrophy Health Index in adolescents and young adults. Muscle Nerve. 2022.
- Mazzella A, Curry M, Belter L, Cruz R, Jarecki J. “I have SMA, SMA doesn’t have me”: a qualitative snapshot into the challenges, successes, and quality of life of adolescents and young adults with SMA. Orphanet J Rare Dis. 2021;16(1):96.



