Dear SMA Community,
Although this year has brought with it many challenges, the SMA community continues to demonstrate its strength and resilience. Cure SMA remains committed to bringing you the information, resources, and support you need as we continue to navigate the COVID-19 pandemic.
Last week, the Advisory Committee on Immunization Practices (ACIP)—a U.S. Centers for Disease Control and Prevention (CDC) committee of healthcare professionals providing guidance on the use of vaccines—recommended Phase 1a of its proposed phased approach for the allocation of vaccines for COVID-19, starting with healthcare workers and residents at long-term care facilities.
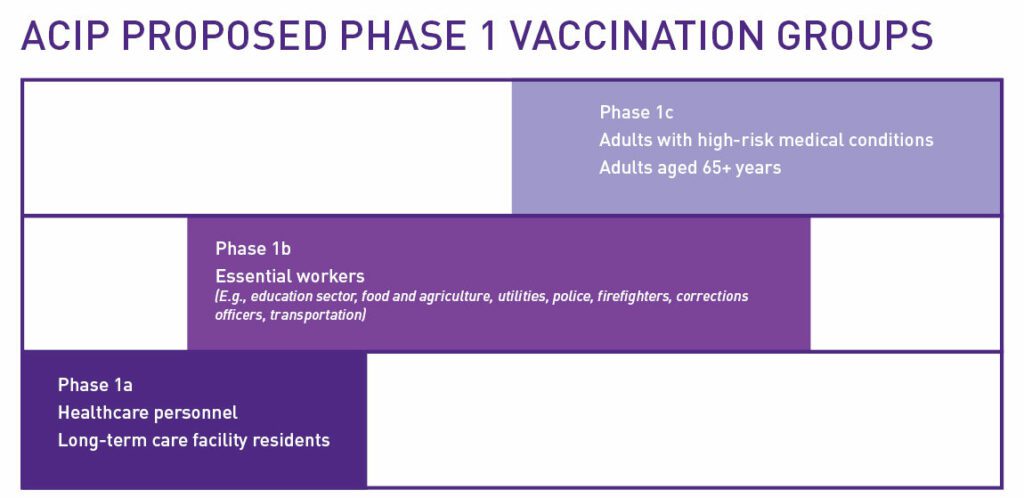
Source: Modified from ACIP COVID-19 Vaccines Work Group graphic
We know that many of you are concerned about vaccine allocation once a vaccination is available. Cure SMA continues to push for priority access to COVID-19 vaccines for the SMA community, advocating directly to the committee and as part of larger healthcare and disability coalitions. ACIP is expected to consider other phases of its allocation framework once COVID-19 vaccines are approved and released.
The ACIP recommendations are a framework that will guide decisions at the state and local level. State and local public health officials will determine distribution of the COVID-19 vaccine in their communities, which is why Cure SMA has also focused our advocacy in states, educating Governors early on in their planning process about the needs of the SMA community. Finally, we developed a resource for the SMA community to use to self-advocate for access to COVID-19 vaccine at the provider level.
ACIP’s initial recommendations were released in advance of the U.S. Food and Drug Administration (FDA) meeting this on December 10. On Thursday, the FDA will discuss the Emergency Use Authorization of the Pfizer-BioNTech vaccine candidate for the prevention of COVID-19 in individuals 16 years of age and older. This is one of the final steps toward approval for the vaccine, at which time the companies will begin the distribution process. Biotechnology company Moderna is also expected to see a review of its vaccine candidate in the coming weeks after a recent filing with the FDA for emergency authorization.
The good news is that vaccines for COVID-19 are on the horizon, and Cure SMA believes that vaccination will be the best defense against this virus. However, there are still a lot of questions to be answered related to the efficacy and safety of available vaccines, who will be eligible to receive the vaccines (i.e., age of recipients), as well as plans around vaccine access. Cure SMA will be closely tracking the available data from vaccine clinical trials and continues to educate and engage with decision-makers to ensure they consider the perspectives and experiences of the SMA community in their recommendations for vaccine distribution.
As we learn more, we will be sharing any FAQs and new resources about approved COVID-19 vaccines on the Cure SMA COVID-19 Information Center—keep an eye out for updates. We also anticipate hosting our next COVID-19 Community Webinar in early 2021, sharing more perspectives and recommendation on COVID-19 vaccination as it specifically relates to the SMA community.
Check out these helpful articles about COVID-19 vaccines from the U.S. Centers for Disease Control and Prevention (CDC).
Finally, while the current progress with vaccines for COVID-19 looks to be very positive, Cure SMA still strongly recommends that the SMA community continue the highest levels of isolation, given we are in the middle of the winter virus season and at the highest peak so far in this pandemic.
Sincerely,
Kenneth Hobby, President, Cure SMA
Dr. Mary Schroth, Chief Medical Officer, Cure SMA


