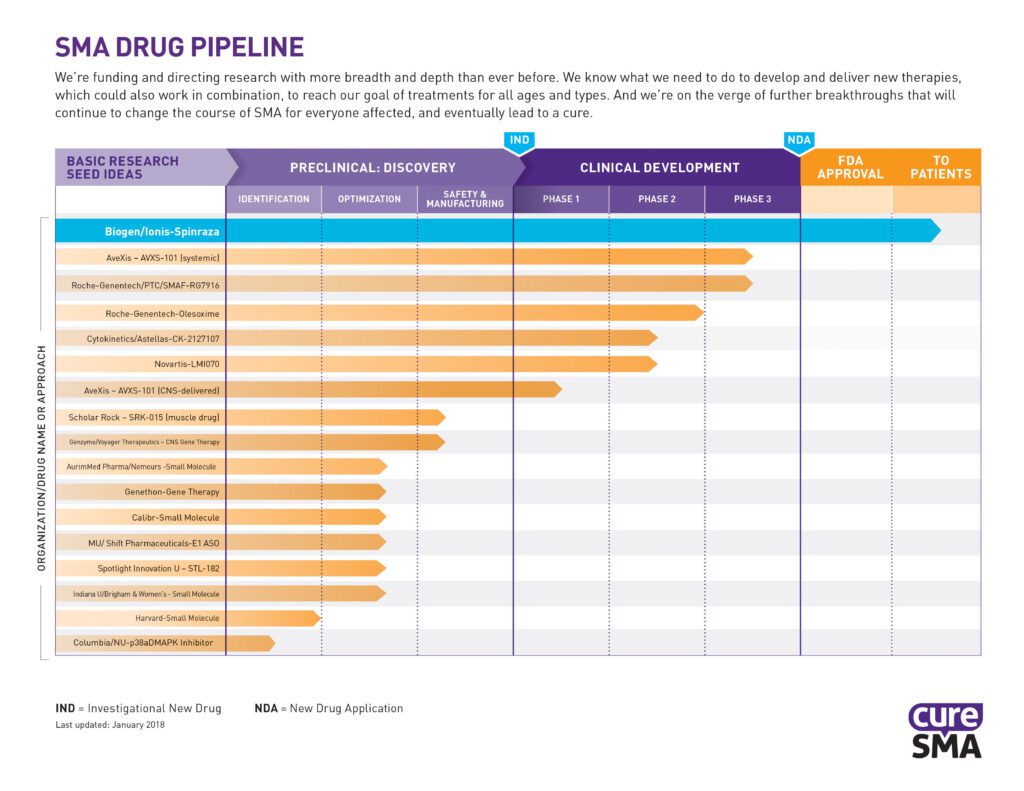
Preliminary Data from FIREFISH Trial in Type 1 SMA Infants Presented at the International Scientific Congress on Spinal Muscular Atrophy
PTC Therapeutics, Inc. recently announced the presentation of early interim data from Part 1, the dose-finding portion of the FIREFISH study. FIREFISH is a two-part seamless, open-label, multi-center study to investigate the safety and efficacy of RG7916 in infants and babies with Type 1 SMA. RG7916 has been safe and well tolerated at all doses […]